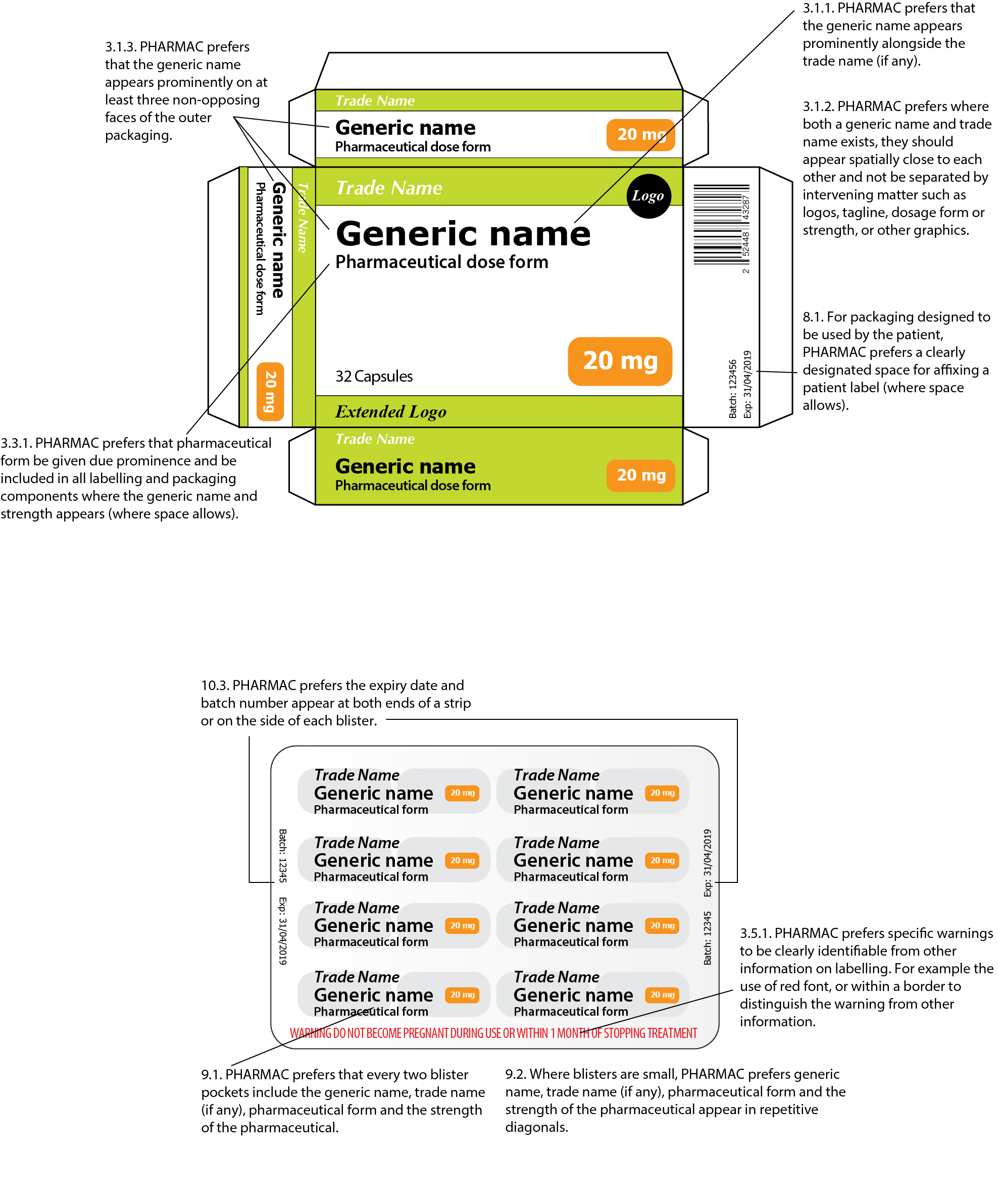
42 cautionary and advisory labels for dispensed medicines
Home - Australian Pharmacist Australian Pharmacist is the Pharmaceutical Society of Australia's monthly journal and is distributed free to all members. It contains pharmacy education and practice features, research papers, health and pharmacy news and information about PSA activities, as well as paid advertising and promotional material. How to use BNF Publications online | About | BNF | NICE Details of these labels can be found in Guidance for cautionary and advisory labels. As these labels have now been applied at the level of the dose form, a full list of medicinal products with their relevant labels would be extensive. This list has therefore been removed, but the information is retained within the monograph.
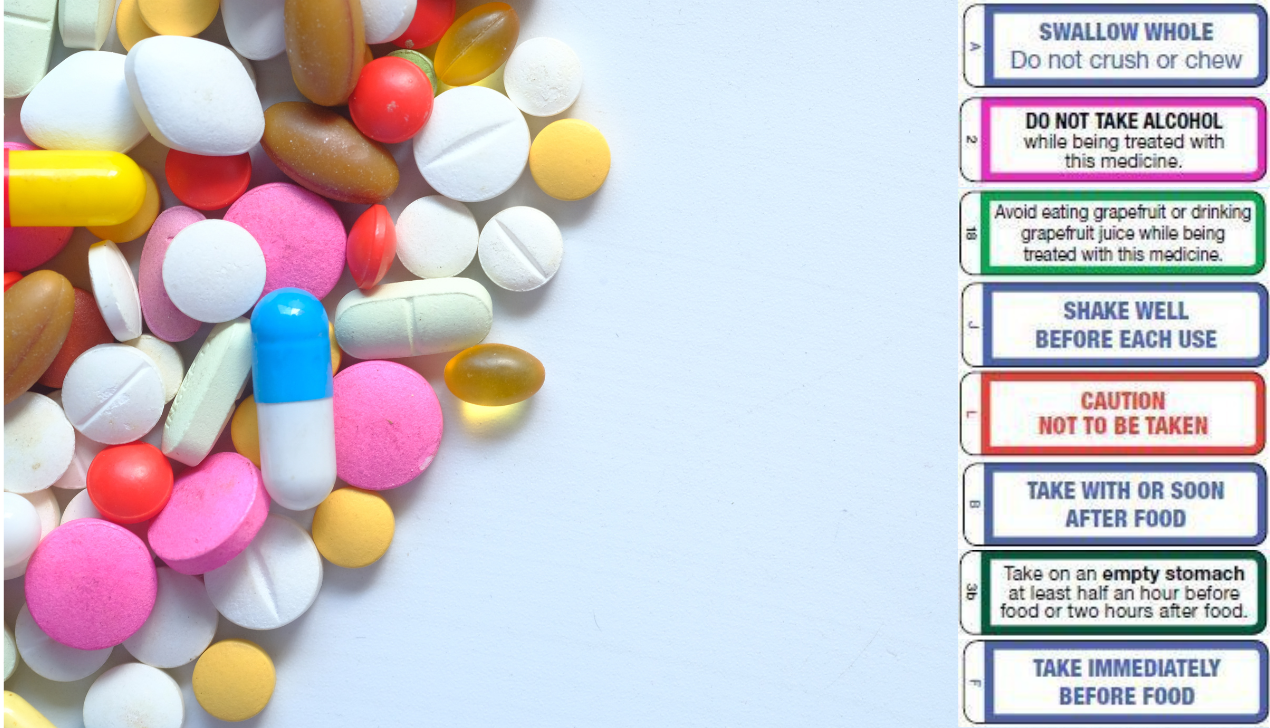
Guidance for cautionary and advisory labels | About | BNF | NICE Wordings which can be given as separate warnings are labels 1–19, 29–30, and 32. Wordings which can be incorporated in an appropriate position in the directions for dosage or administration are labels 21–28. A label has been omitted for number 20; labels 31 and 33 no longer apply to any medicines in the BNF and have therefore been deleted.

Cautionary and advisory labels for dispensed medicines
Poisons Standard February 2022 - Legislation Jan 27, 2022 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; R.A. 7394 - Lawphil 2) Any drug dispensed by filling or refilling a written prescription of a practitioner licensed by law to administer such drug shall be exempt from the requirements of Article 89, except paragraphs (a), (h), (2) and (3), and the packaging requirements of paragraphs (f) and (g), if the drug bears a label containing the name and address of the ...
Cautionary and advisory labels for dispensed medicines. FDA requiring Boxed Warning updated to improve safe use of ... Oct 02, 2020 · In 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient retail and mail-order pharmacies, with alprazolam (38%) being the most common followed by ... R.A. 7394 - Lawphil 2) Any drug dispensed by filling or refilling a written prescription of a practitioner licensed by law to administer such drug shall be exempt from the requirements of Article 89, except paragraphs (a), (h), (2) and (3), and the packaging requirements of paragraphs (f) and (g), if the drug bears a label containing the name and address of the ... Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; Poisons Standard February 2022 - Legislation Jan 27, 2022 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M




























Post a Comment for "42 cautionary and advisory labels for dispensed medicines"