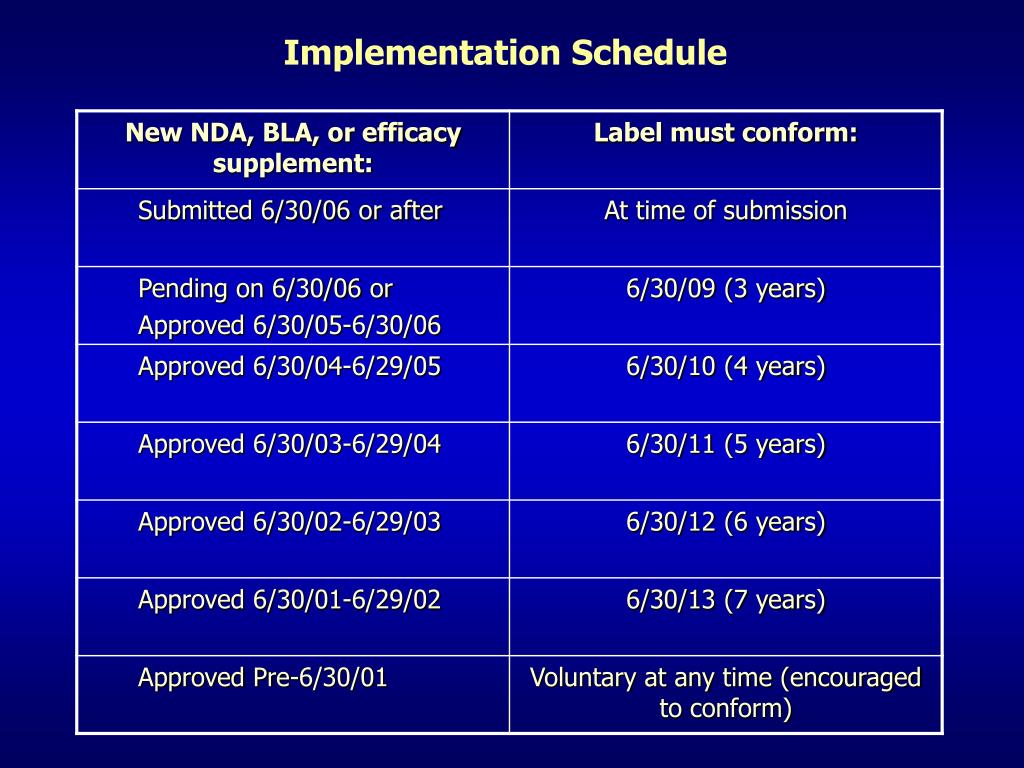
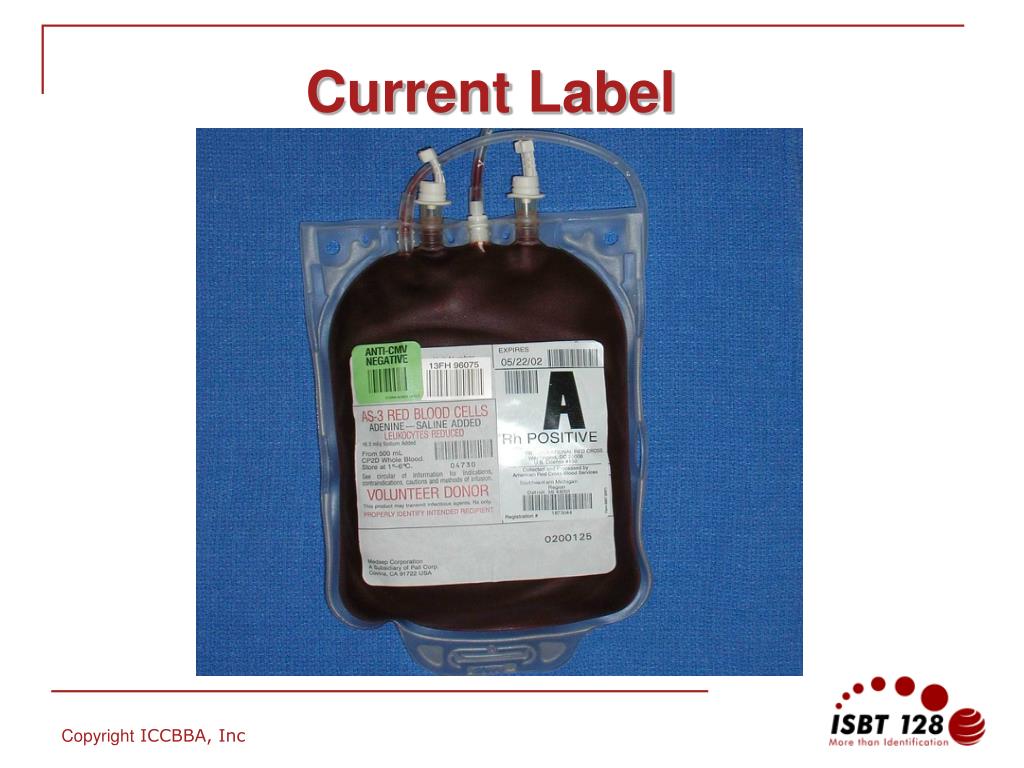
40 fda structured product labels
dailymed.nlm.nih.gov › dailymed › indexDailyMed Sep 15, 2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products ... Tech | Best-In-Class Information-Based Solutions and ... Jun 17, 2022 · Build and submit UDI records electronically in Structured Product Labeling (SPL) format or seek subject-matter expertise. Manage Medical Device Product Data for UDI & Syndication Serving the medical device manufacturing industry in the areas of compliance, data management and regulatory requirements experience.
labels.fda.govFDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.)
Fda structured product labels
› industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Apr 06, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. dailymed.nlm.nih.gov › dailymed › spl-resources-allDailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations. › vaccines › programsIIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ...
Fda structured product labels. › industry › structured-product-labelingNSDE | FDA Mar 31, 2022 · In this section: Structured Product Labeling Resources ... this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov. The information ... › vaccines › programsIIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ... dailymed.nlm.nih.gov › dailymed › spl-resources-allDailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations. › industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Apr 06, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.









Post a Comment for "40 fda structured product labels"